Overview of phosphotransferase system
A novel sugar-phosphorylating system in Escherichia coli has been discovered several years ago. The unique features of this phosphotransferase system (PTS) consist of the use of phosphoenolpyruvate (PEP) as the phosphoryl donor for sugar phosphorylation and the presence of three essential catalytic entities, termed Enzyme I, Enzyme II and HPr (heat-stable, histidine-phosphorylatable protein). Established primary functions of the system include sugar reception, transport and phosphorylation, whereas secondary functions include a lot of ramifications of metabolic and transcriptional regulation. PTS auxiliary proteins such as the fructose repressor, FruR, and the Mlc transcription factor are believed to control transcription of the PTS genetic apparatus as well as of genes encoding central pathways of carbon metabolism in enteric bacteria. Glycolysis, the Krebs cycle, electron transport, the glyoxylate shunt, gluconeogenesis, and possibly the Entner-Doudoroff pathway are all included in these pathways. Genetic evidence has suggested that other processes including the net production of carbon and energy storage sources such as poly-β-hydroxybutyrate and the control of σ-dependent transcription of nitrogen metabolism genes in numerous Gram-negative bacteria are also controlled by the PTS.
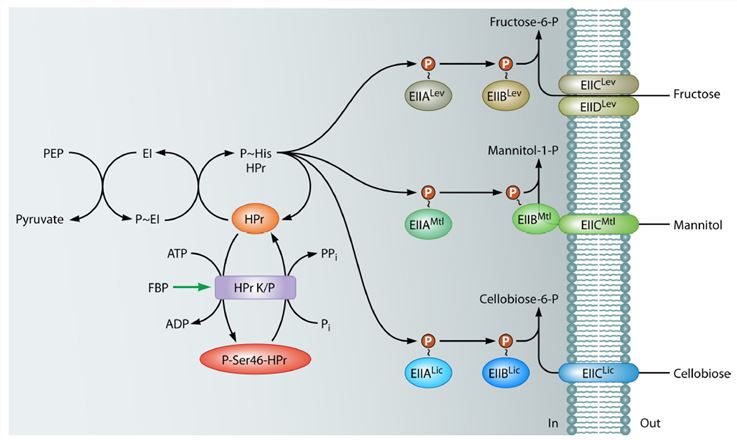
Figure1. phosphorylation cascade formed by the B. subtilis PTS components necessary for the uptake of fructose (PTSLev), mannitol (PTSMtl), and cellobiose (PTSLic).
PST-mediated Regulation by Phosphorylation
Because all cytoplasmic PTS proteins or membrane-associated hydrophilic PTS domains undergo transient phosphorylation, it is not surprising that lots of numerous regulatory functions of the PTS are mediated by phosphoryl transfer to the target proteins. Identical to the case for PTS proteins, all presently known PTS-regulated target proteins also become phosphorylated at histidine (phosphoamidate) or cysteine (thiophosphate) residues. There are two major factors which were so far reported to affect the phosphorylation state of the PTS proteins in Enterobacteriaceae and probably most other bacteria. First, the uptake of an efficiently metabolized PTS sugar, such as glucose, and its concomitant phosphorylation lead to dephosphorylation of the PTS components. Second, changes in the ratio of PEP to pyruvate, the two compounds which are the substrates and products of the autophosphorylation of E1, also affect the phoapgorylation state of the PTS proteins. Finally, α-ketoglutarate, which accumulates in E. coli cells exposed to nitrogen-limiting conditions, inhibits the autophosphorylation activity of E1.
- Proteins containing their own PTS-Recognized Phosphorylation sites
It might be expected that the development of a phosphorylation site recognized by PTS components would be the most straightforward and simplest way to render a protein “PTS controlled.” However, there is so far only one well-suited example in which a protein develops a specific regulatory site (as opposed to the enzymatic sites described above) for phosphorylation by a PTS protein. This is glycerol kinase (GlpK) from firmicutes.
- Proteins Containing a PTS Component Fused to the N or C Terminus
Another mechanism of PTS-mediated regulation that developed during bacterial evolution was the fusion of a PTS component to a target protein. In most cases the phosphorylatable His or Cys is conserved in the regulatory PTS domain and can therefore get phosphorylated by the proteins of the PTS phosphorylation cascade. For some proteins it was not an entire PTS domain that was fused to the target polypeptide but only a relatively small fragment containing the phosphorylatable histidine or cysteine. Phosphorylation or dephosphorylation of the PTS domain induces structural changes in the target protein, which lower or stimulate its activity. The PTS domain-containing transcription activators are mainly found in firmicutes and in actinobacteria but are less frequent in proteobacteria. Some of these bacteria possess transcription activators which can contain up to three EIIA and EIIB domains. LacS from S.thermophilus contains an EIIAGlc-like domain fused to its C-terminus. This natural hybrid protein catalyzes slow H+/lactose symport as well as fast lactose/galactose counterflow. Otherwise, several proteins were found to contain an HPr-like domain fused either to their N or C termini. The presence of an HPr-like domain at the N terminus of an NtrC-type regulator with a C-terminal DNA binding domain (but without EII domains) in Clostridium acetobutylicum ATCC 824 suggested a possible cross talk between this transcription regulator (called HprR) and the PTS, with the HPr-like domain functioning as the receiver module. Finally, in Pasteurella multocida Pm70 and several other P. multocida strains as well as in Actinobacillussuccinogenes 130Z, an entire EIIBGlc-like domain is fused to another glycolytic enzyme, namely, triosephosphate isomerase. The phosphorylatable Cys is conserved in all these proteins, suggesting that their activity might be controlled by PTS mediated phosphorylation in response to the presence or absence of glucose, which in most Pasteuriaceae is taken up via a PTS.
- Proteins Containing a Specific PTS-Recognized Phosphorylation Domain, the PRD
In the course of evolution, bacteria must have produced peptide composed of about 85 amino acids forming principally two slightly twisted antiparallel α-helices connected by an unstructured loop. The second α-helix contains a histidine, which is arranged in such a way that it can be phosphorylated by at least two different PTS proteins. The well-studied antiterminator LicT of B. subtilis controls the expression of the bglPH and bglS genes, which encode the PTS permease and catabolic enzymes for the uptake and metabolism of β-glucosides. The phosphorylation of PRDs in transcription activators is more variable. In some transcription activators, such as MtlR, only one PRD becomes phosphorylated by EI and HPr, whereas in others, such as LicR, all four histidines of the two PRDs are phosphorylated by the general PTS components and mutation of either one of these histidines leads to a loss of function. Mga, one of the regulators of Streptococcus pyogenes virulence genes, was also reported to be phosphorylated by EI and HPr. Phosphorylation was found to occur at a specific histidine in the central part of Mga, which was claimed to resemble PRDs.
References:
- Jr S M. The bacterial phosphotransferase system: structure, function, regulation and evolution.Journal of Molecular Microbiology & Biotechnology. 2001, 3(3):325-327.
- Deutscher J, et al. The bacterial phosphoenolpyruvate: carbohydrate phosphotransferase system: regulation by protein phosphorylation and phosphorylation-dependent protein-protein interactions. Microbiology & Molecular Biology Reviews Mmbr. 2014, 78(2):231-56.





